Ruwisch, J., Sehlmeyer, K., Roldan, N., Garcia-Alvarez, B., Perez-Gil, J., Weaver, T.E., Ochs, M., Knudsen, L., Lopez-Rodriguez, E.: Am J Resp Cell Mol. 2020;62(4):466-78.
To access the full article, click here:

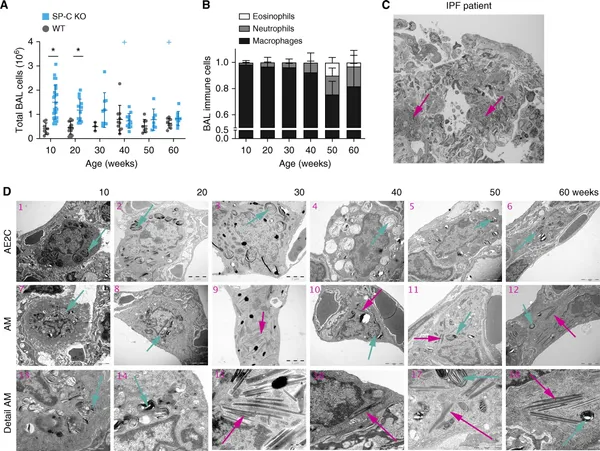
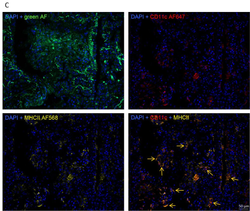
Surfactant protein (SP)-C deficiency is found in samples from patients with idiopathic pulmonary fibrosis, especially in familial forms of this disease. We hypothesized that SP-C may contribute to fibrotic remodeling in aging mice and alveolar lipid homeostasis. For this purpose, we analyzed lung function, alveolar dynamics, lung structure, collagen content, and expression of genes related to lipid and cholesterol metabolism of aging SP-C knockout mice. In addition, in vitro experiments with an alveolar macrophage cell line exposed to lipid vesicles with or without cholesterol and/or SP-C were performed. Alveolar dynamics showed progressive alveolar derecruitment with age and impaired oxygen saturation. Lung structure revealed that decreasing volume density of alveolar spaces was accompanied by increasing of the ductal counterparts. Simultaneously, septal wall thickness steadily increased, and fibrotic wounds appeared in lungs from the age of 50 weeks. This remarkable phenotype is unique to the 129Sv strain, which has an increased absorption of cholesterol, linking the accumulation of cholesterol and the absence of SP-C to a fibrotic remodeling process. The findings of this study suggest that overall loss of SP-C results in an age-dependent, complex, heterogeneous phenotype characterized by a combination of overdistended air spaces and fibrotic wounds that resembles combined emphysema and pulmonary fibrosis in patients with idiopathic pulmonary fibrosis. Addition of SP-C to cholesterol-laden lipid vesicles enhanced the expression of cholesterol metabolism and transport genes in an alveolar macrophage cell line, identifying a potential new lipid–protein axis involved in lung remodeling.