Avcibas R., Vermul A., Gluhovic V., Boback N., Arroyo R., Kingma P.S., Isasi-Campillo M., Garcia-Ortega L., Griese M., Kuebler W.M., Ochs M., Lauster D., and Lopez-Rodriguez E. Am J Physiol-Lung Cell Mol Physiol 2024 326:5, L524-L538
To access the full article, click here:

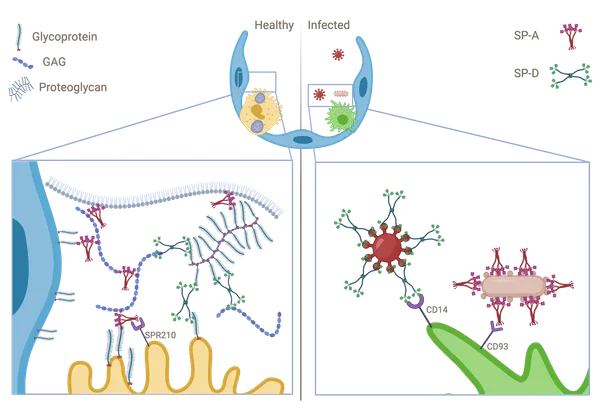
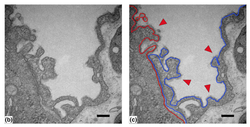
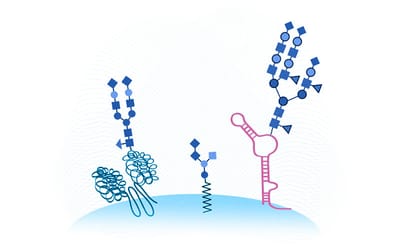
Lung surfactant collectins, surfactant protein A (SP-A) and D (SP-D), are oligomeric C-type lectins involved in lung immunity. Through their carbohydrate recognition domain, they recognize carbohydrates at pathogen surfaces and initiate lung innate immune response. Here, we propose that they may also be able to bind to other carbohydrates present in typical cell surfaces, such as the alveolar epithelial glycocalyx. To test this hypothesis, we analyzed and quantified the binding affinity of SP-A and SP-D to different sugars and glycosaminoglycans (GAGs) by microscale thermophoresis (MST). In addition, by changing the calcium concentration, we aimed to characterize any consequences on the binding behavior. Our results show that both oligomeric proteins bind with high affinity (in nanomolar range) to GAGs, such as hyaluronan (HA), heparan sulfate (HS) and chondroitin sulfate (CS). Binding to HS and CS was calcium-independent, as it was not affected by changing calcium concentration in the buffer. Quantification of GAGs in bronchoalveolar lavage (BAL) fluid from animals deficient in either SP-A or SP-D, showed changes in GAG composition, and electron micrographs showed differences in alveolar glycocalyx ultrastructure in vivo. Taken together, SP-A and SP-D bind to model sulfated glycosaminoglycans of the alveolar epithelial glycocalyx in a multivalent and calcium independent way. These findings provide a potential mechanism for SP-A and SP-D as an integral part of the alveolar epithelial glycocalyx binding and interconnecting free GAGs, proteoglycans and other glycans in glycoproteins, which may influence its glycocalyx composition and structure.